- Your cart is empty
- Continue Shopping
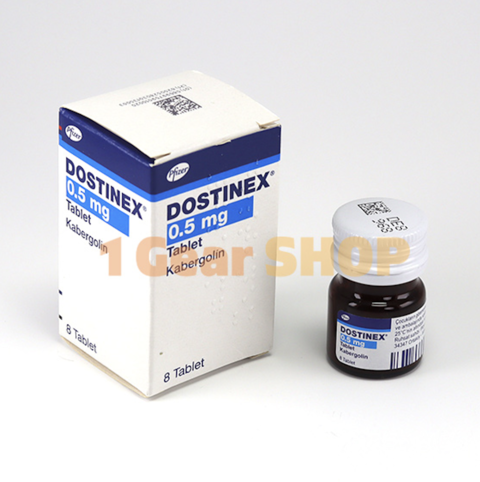
Buy Original Dostinex (Cabaser) Pfizer Cabergoline 8 tabs x 0.5 mg Online at 1gearshop.com
Active Substance: Cabergoline.
Product Dose: 0.5 mg/1tab.
Drug Class: Prolactin Control.
Pharmacotherapeutic Group: Dopamine Agonists.
Brand: Pfizer.
Count: 8 tablets.
What is Dostinex (Cabaser) Pfizer Caberoline ?
Dostinex (Cabaser), a derivative of ergoline with potent dopamine D2 receptor agonist properties, acts swiftly (within 3 hours of intake) and maintains its effects for a prolonged period (lasting up to 7-28 days). The reduction in PRL (prolactin) levels correlates with the dosage, both in terms of intensity and duration of action.
Dostinex (Cabaser) finds utility in alleviating symptoms associated with Parkinson’s disease. It contains Cabergoline as its active component, which mimics the action of dopamine, a neurotransmitter deficient in Parkinson’s patients.
Dostinex’s Pharmacokinetic
Dostinex pharmacokinetic and metabolic characteristics have been scrutinized in healthy volunteers of both genders, as well as in female hyperprolactinemic and parkinsonian patients. Following oral administration, radioactivity from the labeled compound is swiftly absorbed from the gut, with peak plasma levels observed between 0.5 to 4 hours. Approximately 18-20% and 55-72% of the administered radioactive dose (3H-cabergoline/14C-cabergoline) is recovered in urine and feces, respectively, ten days post-administration. Studies with the non-radioactive product have also confirmed minimal urinary excretion of unchanged Cabaser. The compound’s elimination half-life, estimated via urinary excretion rates, is prolonged (63-68 hours in healthy volunteers, 79-115 hours in hyperprolactinemic patients). Food intake does not notably impact Dostinex absorption or disposition; however, consuming it with meals may enhance tolerability, a common trait observed with dopaminergic agents.
Dostinex (Cabaser) Therapeutic Indication:
Dostinex (Cabaser) is recommended as second-line therapy for patients intolerant or unresponsive to non-ergot compounds in the treatment of Parkinson’s disease, either as monotherapy or adjunctive therapy alongside levodopa plus a dopa-decarboxylase inhibitor.
Dostinex (Cabaser) Dosage and Administration:
Dose response, concerning both efficacy and adverse effects, appears to be individualized. Therefore, initiating therapy with low doses (1 mg daily) and gradually titrating upwards is advised. Concurrent levodopa dosage may be reduced gradually as Dostinex dosage is increased, until an optimal balance is achieved. Due to its extended half-life, dosage increments of 0.5-1 mg should occur weekly (initial weeks) or bi-weekly, until reaching optimal doses. The recommended therapeutic dose for Parkinson’s disease patients is 2 to 3 mg/day, administered as a single daily dose.
Use in Children:
The safety and efficacy of Dostinex have not been established in pediatric populations, as Parkinson’s disease is not prevalent in this demographic.
Adverse Reactions:
Common adverse events associated with Dostinex include gastrointestinal disturbances, peripheral edema, constipation, dyspepsia, gastritis, vomiting, dizziness, dyskinesia, confusion, and hallucinations.
Dostinex (Cabaser) Contraindications:
Cabaser should not be administered to individuals with hypersensitivity to the drug or any of its excipients, or to those with a history of pulmonary, pericardial, or retroperitoneal fibrotic disorders. Pre-treatment echocardiography is recommended for long-term therapy to rule out cardiac valvulopathy. Caution is warranted in patients with severe cardiovascular disease, Raynaud’s syndrome, peptic ulcer or gastrointestinal bleeding, or a history of serious mental disorders, as well as in those with rare hereditary conditions such as galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption.
Pregnancy and Lactation:
Observational data from a twelve-year study on pregnancy outcomes following cabergoline therapy indicate that approximately 6.6% of pregnancies resulted in major congenital malformations or abortion, with neonatal abnormalities observed in 23 out of 258 infants. However, the precise risk compared to the general population remains indeterminate due to the absence of a control group. Long-term developmental outcomes of infants exposed to intra-uterine cabergoline are not well-established.
Storage Temperature:
Cabaser should be stored at room temperature (15-25°C), shielded from sunlight and moisture.
| Brands | |
|---|---|
| Shipping Zones | |
| Active Substances | |
| Dose | |
| Count | |
| Form | |
| Use |
Only logged in customers who have purchased this product may leave a review.







Reviews
There are no reviews yet.